Cognitive control of behavior and hippocampal information processing without medial prefrontal cortex
For over 20 years grant reviewers and other critics have asserted that the Room+Arena- active place avoidance task variant must depend on mPFC and that either a) it is not a task that requires cognitive control or b) that if it does require cognitive control that is because it must depend on the mPFC not the hippocampus,. We’ve demonstrated the sensitivity to hippocampal dysfunction multiple times. This new paper, published in eLife should settle the matter concerning the mPFC once and for all.
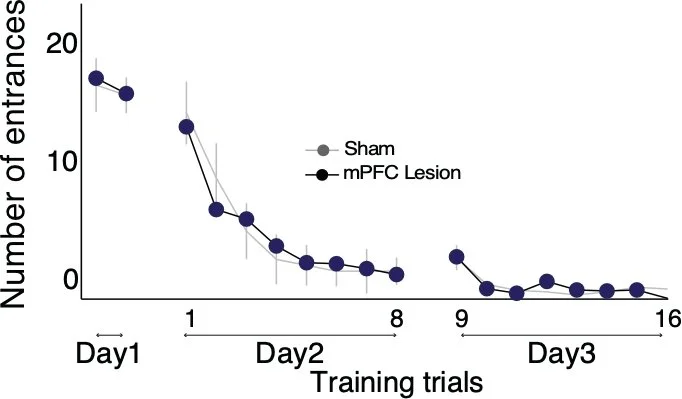
We lesion mPFC, and despite confirming the lesion and effects beyond the lesion, there is no effect on behavior. We detect lesion effects on hippocampal single unit action potential discharge but no effect on the representational switching between hippocampal discharge judiciously signaling room and arena locations , i.e. there was no effect of mPFC lesion of the sine qua non of cognitive control.
What almost no one knows is that over 20 years ago, before 2003, I tested whether mPFC lesions would impair R+A- place avoidance and of course the lesion did not. I even tested this in collaboration with Etienne Save and Bruno Poucet at CNRS in Marseille, France (I think Vincent Hok was their trainee at the time and did the experiments). These were pilot studies and we never published the data. I had gotten used to the very confident reviewer assertions and also every new postdoc’s assertion that R+A- active place avoidance had to be a mPFC dependent task. Happily EunHye and Kally (now both at Columbia, Psychiatry) decided to do a definitive study knowing there was likely to be a “negative” result that no one would believe. They did the experiments carefully and comprehensively and the results were definitive at the levels of behavior, hippocampal representations of spatial information, and the lesion effects. Even so, this was hard to publish (see the eLife reviews), likely because it challenges the belief that mPFC is responsible for expressions of all cognitive control.
EunHye and Kally worked extra hard on this paper and at times it seemed thankless — yup, rigorous science is hard! Congrats to them along with much gratitude for bringing clarity to the matter for the lab and the field. Thanks also to the eLife editors, and the reviewers for managing a fair review process, and for pushing us to make super clear the ideas and controversies that the paper addresses.
Garrett’s got SMART
Spatial Memory and Allocentric Reinforcement Training
See comment in the Transmitter:
What are mechanisms? Unpacking the term is key to progress in neuroscience
Mechanism is a common and powerful concept, invoked in grant calls and publication guidelines. But scientists use it in different ways, making it difficult to clarify standards in the field. We asked nine scientists to weigh in.
Figure from the commentary: Schematic of a common electrical circuit the “single pole double throw switch” illustrates how so-called causal investigations can be ill-conceived depending on the assumptions about the underlying circuit architecture. A) Observing this position of switch 1, one might hypothesize it turns on the light, and B) confirm this inference by manipulating switch 1 to the other position. However, when switch 2 changes position as in C,D), the opposite conclusions will be reached about switch 1 (and 2). It would be wrong to conclude that switch 1 does not control the light, because it does, and although manipulating a single switch cannot reveal its function, the circuit logic is readily understood by observing the correlations between switches 1 and 2 and the on/off state of the light bulb. Note also that removing either switch would show that it is necessary for the functioning of the circuit, but not reveal the operational logic.
The next chapter in the story of how PKMzeta maintains memory for months …
KIBRA anchoring the action of PKMzeta maintains the persistence of memory
Image of KIBRA (green) and PKMzeta (red) illustrating their sites of interaction. The KIBRA amino acid sequence at the PKMzeta binding site is indicated in mustard. Expressing just the peptide (K-ZAP) inhibits the KIBRA-PKMzeta interaction, The small molecule inhibitor zeta-stat (gray) binds to an allosteric pocket (yellow) on PKMzeta to also prevent the KIBRA-PKMzeta interaction. Neither K-ZAP or zeta-stat had effects on LTP or memory persistence in mice in which PKMzeta was genetically deleted, demonstrating their selective effects on PKMzeta. PKMzeta and the other atypical PKC called PKCiota/lambda have very similar kinase domains but mutating two PKMzeta amino acids (purple, Proline 291 and Phenylalanine 297) to those in PKCiota/lambda (Glutamine 478 and Serine 484) reduces the likelihood of the KIBRA-PKMzeta complex and prevents zeta-stat from having an effect. Credit: Changchi Hsieh
Extremely proud to report this recently published work, the latest in the tremendously rewarding and joyous collaboration with the Sacktor lab at SUNY Downstate. We find that KIBRA targets PKMzeta’s kinase activity to activated synapses where the KIBRA-PKMzeta complex acts as a persistent synaptic tag to perpetuate potentiated synapses for at least a month, which is several times longer than the existence of the individual protein components. KIBRA is selectively positioned in these activated synapses. PKMzeta then attaches to the KIBRA-synaptic-tag and keeps those synapses potentiated, stronger than at baseline. This allows the synapses to stick to newly made KIBRA, attracting more newly made PKMzeta, which acts as a persistent synaptic tag.
The finding explains two observations that have puzzled me for almost a decade.
1) How viral over expression of PKMzeta strengthens weak memories rather than degrades them by saturating synapses (Shema et al., 2021, Science DOI: 10.1126/science.1200215)
2) How genetic deletion of PKMzeta is compensated by PKCiota/lambda (Tsokas et al., 2016 eLife DOI: 10.7554/eLife.14846)
The persistent synaptic tagging mechanism we found is analogous to how new planks replace old planks to maintain Theseus’s Ship for generations, and allows memories to last for years even as the proteins maintaining the memory are replaced, Francis Crick intuited this Theseus’s Ship mechanism, and he even predicted the role for a protein kinase.
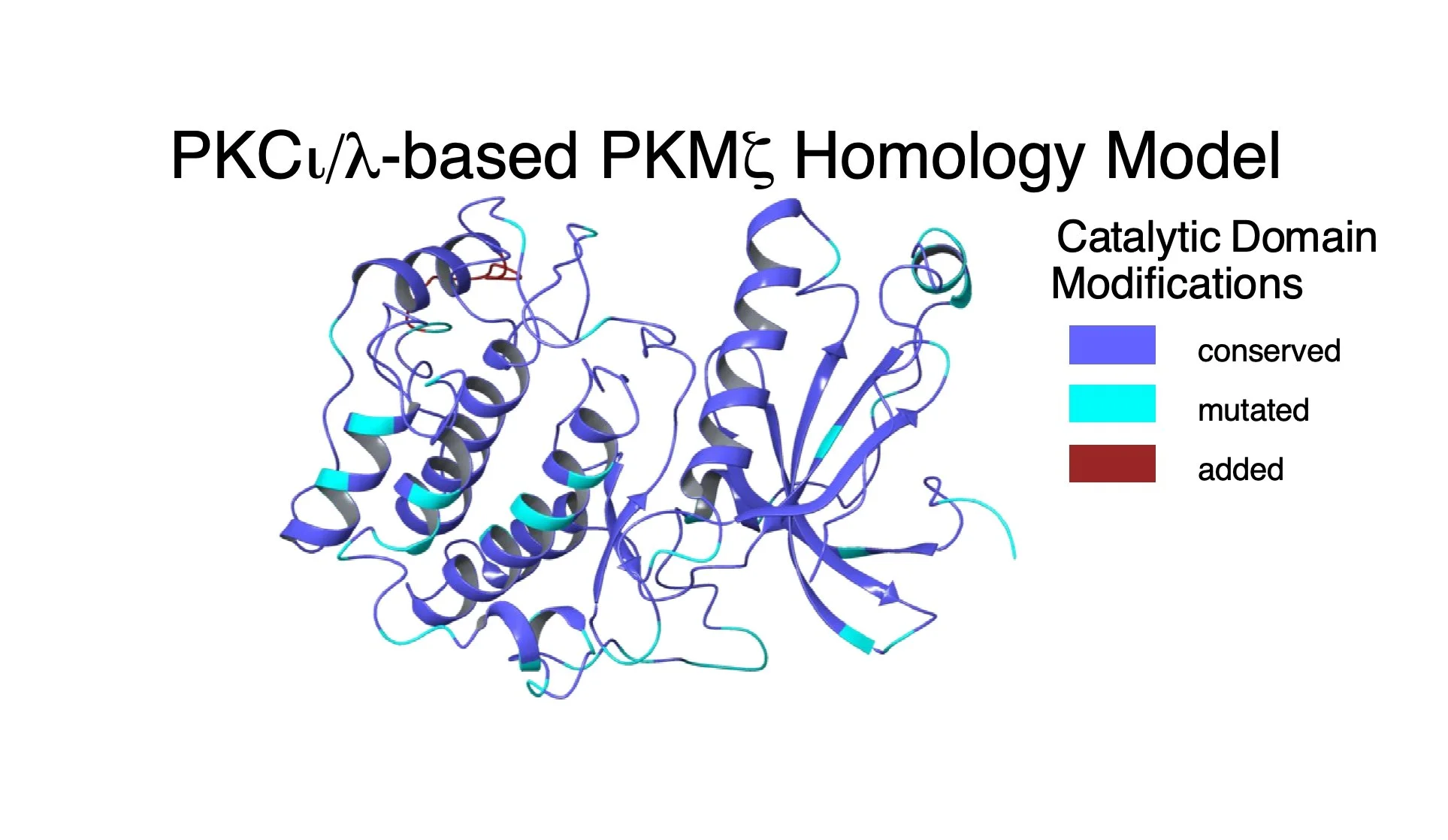
PKMzeta and PKCiota/lambda derive from duplication of a single invertebrate atypical PKC gene. Sometime after jawless fish, the zeta isoform evolved an internal promoter at the hinge region between the regulatory and kinase domains of the aPKC. The mammalian PKMzeta is thus transcribed from this internal promotor and having no regulatory domain, it is constitutively active once it is synthesized. Image: Based on the known structure of the PKCiota/lambda kinase domain, a homology model of PKMzeta was constructed with the conserved, mutated and added amino acids indicated. Notice that the two kinases are very similar, which is helps to explain why genetic deletion of PKMzeta is readily compensated by increased PKCiota/lambda. Credit: Tom Ko
In Levy et al 2023 we used Video S2, an animation of a jumbotron display of the message “Game Over” to help readers get an intuition for how variable patterns of activity can produce a reliable signal when the correlations between the components of the signal define low-dimensional subspaces within which the activity is organized. I use this in lectures and have been told it helps develop the intuition that can be illusive. Here’s the video.
Dynamic jumbotron patterns as an example of an ensemble, non-linear manifold representation of an invariant message. In this video of a jumbotron display the “Game Over” message is constant but which lights, the number of lights, where the lights turn on and off, which lights are on and off together and such, are all different, despite the message being the same. How CA1 activity represents a cylinder and a box lead us to consider that a manifold representation with no requirement for either single cell or ensemble stationarity is the appropriate conceptual framework for understanding how hippocampal activity represents spatial information. We analyzed the 24-s video as a 180 × 320 (57600) ensemble time series across 720 frames (time steps). The PR that estimates the dimensionality is 600 (∼1%). Projecting the 57600-D activity vectors into the 3-D non-linear subspace with the IsoMap algorithm (left), yields a reconstruction error increase of merely 5% relative to the reconstruction error using a 550-D IsoMap subspace, quantifying that most of the observed variance is captured by the 3 non-linear dimensions. A similar projection has been performed into the 3-D subspace using PCA (right). The two videos illustrate that the jumbotron’s “Game Over” message occupies a very limited portion of the 3-D subspaces the axes of which are defined by particular jumbotron patterns of “Game Over.” Excerpted from Levy et al., 2023 Cell Reports.
A manifold neural population code for space in hippocampal coactivity dynamics independent of place fields
Photo credit: Chris Strickland
CA1 place cells were recorded while mice explored a box and a cylinder using a standard remapping paradigm. Although standard remapping features of firing field changes were observed, the seconds-scale cofiring relationships between pairs of cells tended not to change between the two environments. Instead, the cells whose discharge was negatively correlated to many other cells expressed environment-specific anti-cofiring, such that the specific anti-cofiring subset predicts the environment. These observations compel us to reinterpret the remapping phenomenon as a reregistration of an essentially invariant temporally-organized manifold of neural population coactivity to salient features of each environment, which we hypothesize are driving the anti-cofiring cells. The dynamics and statistics of these observations are remarkably similar to the dynamics that organize collective behavior in flocks of birds like the starling murmuration in the photograph.
This is result of an amazing synergistic collaboration between Eliott Levy and Simón Carrillo-Segura when they were non-overlapping Ph.D. students. The collaboration continues.
Eliott Levy
Simón Carrillo-Segura
How do humans learn and know? Previously, it was assumed that neurons respond to external stimuli to represent them, but an equally plausible model asserts that neuronal activity is fundamentally internally-organized and instead fit to external features of the world. Studies of spatially-tuned cells in various cortices that investigate how experience changes neuronal information processing (in addition to storing memory), and how encoding and recollecting experience is coordinated, will be reviewed.
André’s SfN 2023 Special Lecture
André’s interview on the Synaptic podcast. This might be one way you can get to really know André, or at least hear stories of his days in grade school, high school, and in the big leagues of science school …
2023- 09-08
Garrett Blair was awarded an NRSA F32 grant.
This research grant from the National Institute of Health will fund research investigating hippocampal-cortical interactions during cognitive control behavior.
2023-05-17
André featured in PBS NOVA series
Neuroscientists discover the tricks and shortcuts the brain takes to help us survive
https://www.pbs.org/wgbh/nova/video/your-brain-perception-deception/
Beyond memory, learning to learn
There are countless studies of the neurobiology of memory, how neurons store the information gained from experience so it can be recalled. But as any educator knows, merely recollecting the information we learn in school is hardly the point of an education.

Dentate Spikes Control: Dino’s Latest
Dino explores dentate spikes and external control of hippocampal function.

Head-direction cell paper with Jim Ranck - Neuron Cover
Exciting to see the Cover image of today's issue of Neuron featuring EunHye's artwork - adaptation of a photo inspired by etak navigation of the Pacific Islanders!